space
EU project
PRECISION ONCOLOGY OF SPATIAL IMMUNE ESCAPE MECHANISMS IN OVARIAN CANCER

Tumor progression is dependent on the ability of malignant cells to escape the recognition and attack by the host immune system
The development of more efficient cancer immunotherapies has been hampered by the perplexity of immune escape mechanisms.
We hypothesize that tumor genetic drivers determine the immune escape mechanisms, and that these mechanisms can be exploited to develop more effective immunotherapies for patients with high-grade serous ovarian cancer (HGSC), the most common and lethal ovarian cancer.
Our project in brief
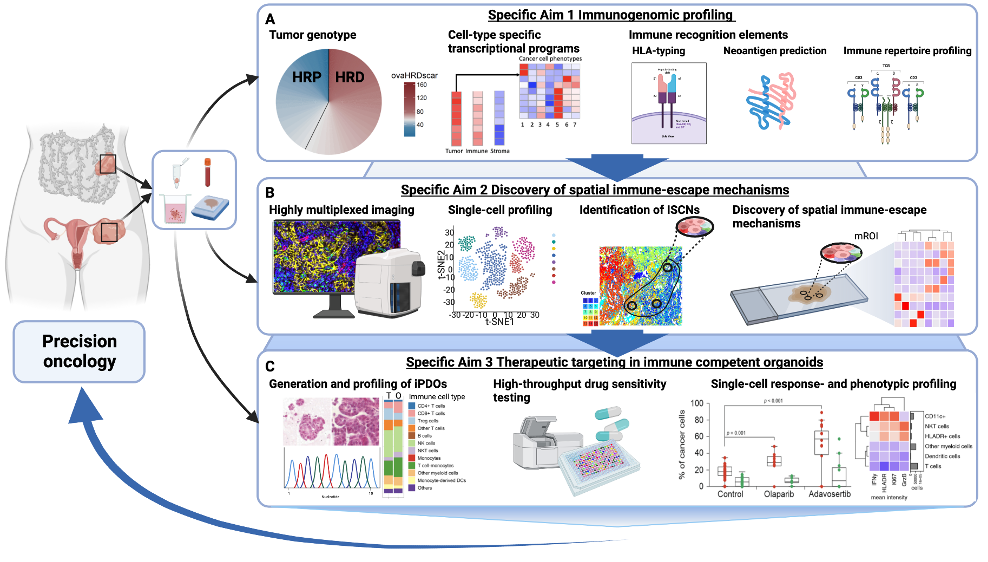
An optimized algorithm to detect homologous recombination DNA repair deficiency
We have developed an optimized algorithm to detect homologous recombination DNA repair deficiency, enabling clinically meaningful stratification of the complex HGSC genotypes (Perez-Villatoro et al NPJ Precision Oncology 2022).
We will define the immunogenicity of the HGSC genotypes via profiling tumor somatic mutations and neoantigens, cell-type specific gene expression patterns, and T/B cell receptor diversities using altogether >600 HGSC samples.
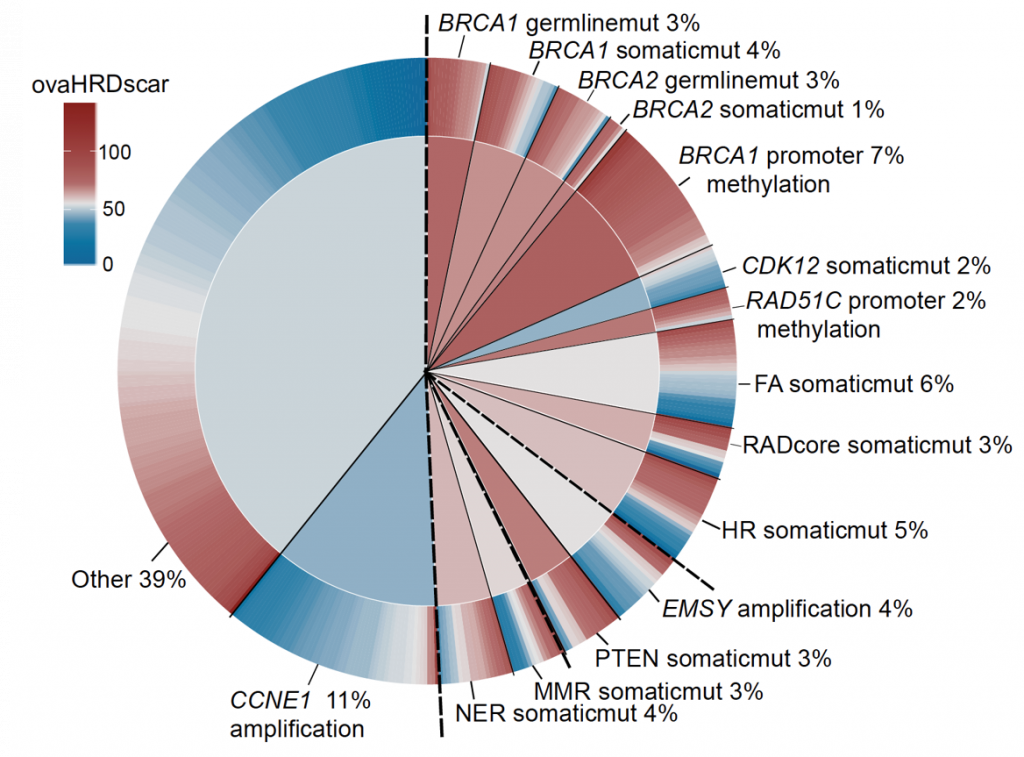
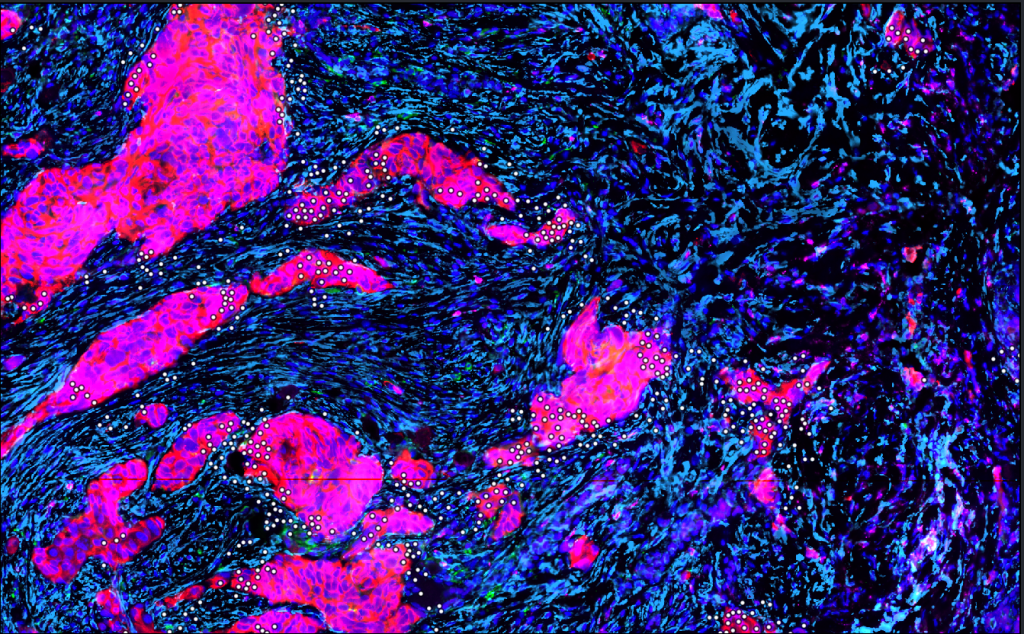
Highly multiplexed technology
We will use a cutting-edge highly multiplexed technology and advanced image analyses (Launonen et al, Nature Communications 2022) to reveal the single-cell spatial landscapes of 200 prospectively collected HGSCs.
Single-cell data analyses and transcriptomic profiling
To uncover the spatial biology underpinning immune escape in HGSCs, we will perform pioneering spatial analyses on the single-cell data and integrate these to immunogenomic and clinical data using machine learning. Via spatial transcriptomic profiling we will discover the detailed immune escape mechanisms of the HGSC genotypes.
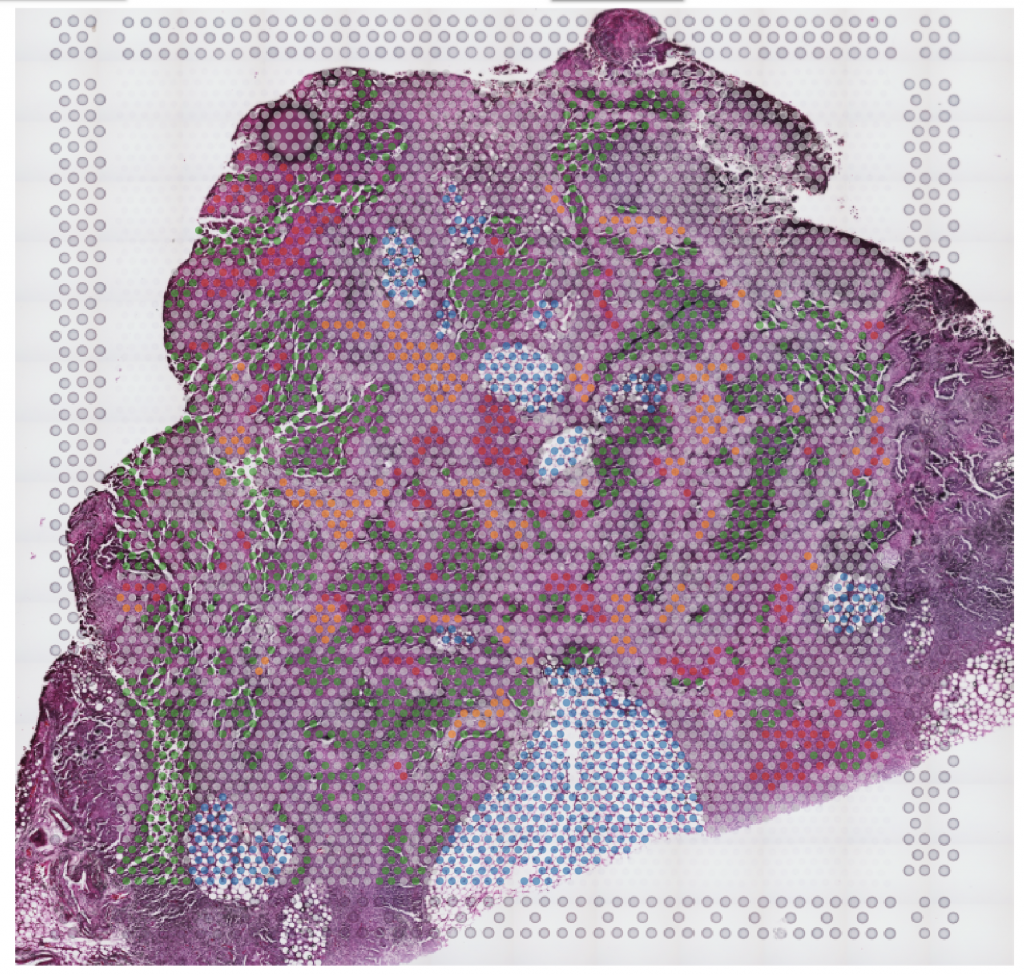
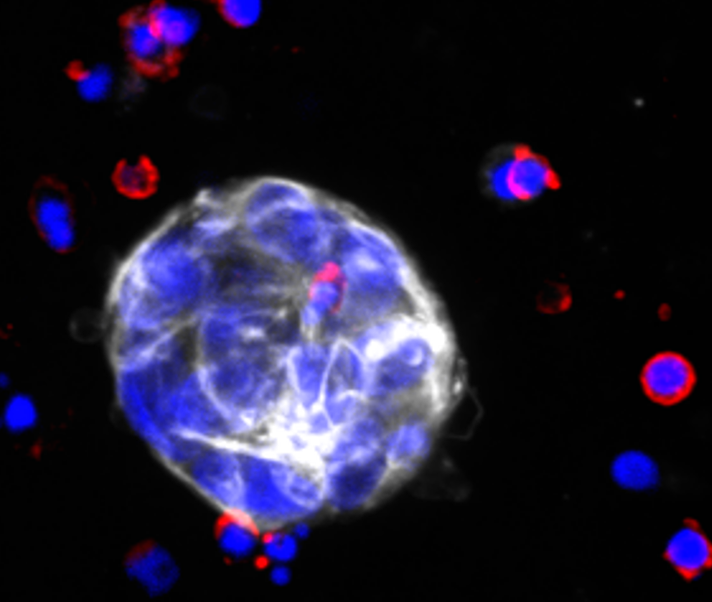
Immune-competent patient-derived organoids (iPDOs)
For therapeutic targeting of the immune-escape mechanisms, we have developed a groundbreaking method to establish immune-competent patient-derived organoids (iPDOs), which faithfully recapitulate the patients’ tumors. Using our high-throughput iPDO platform, we will test mechanism-specific immunotherapeutic approaches and capture the treatment responses at single-cell resolution.